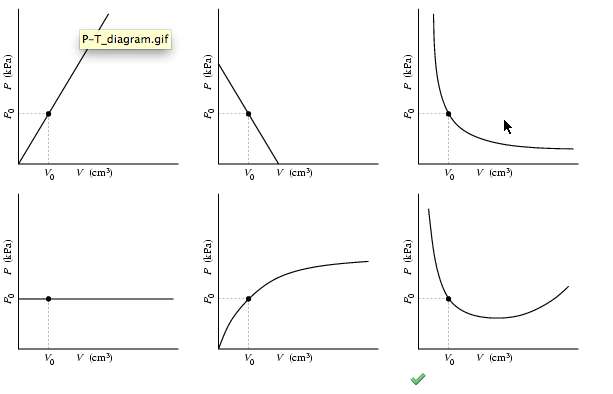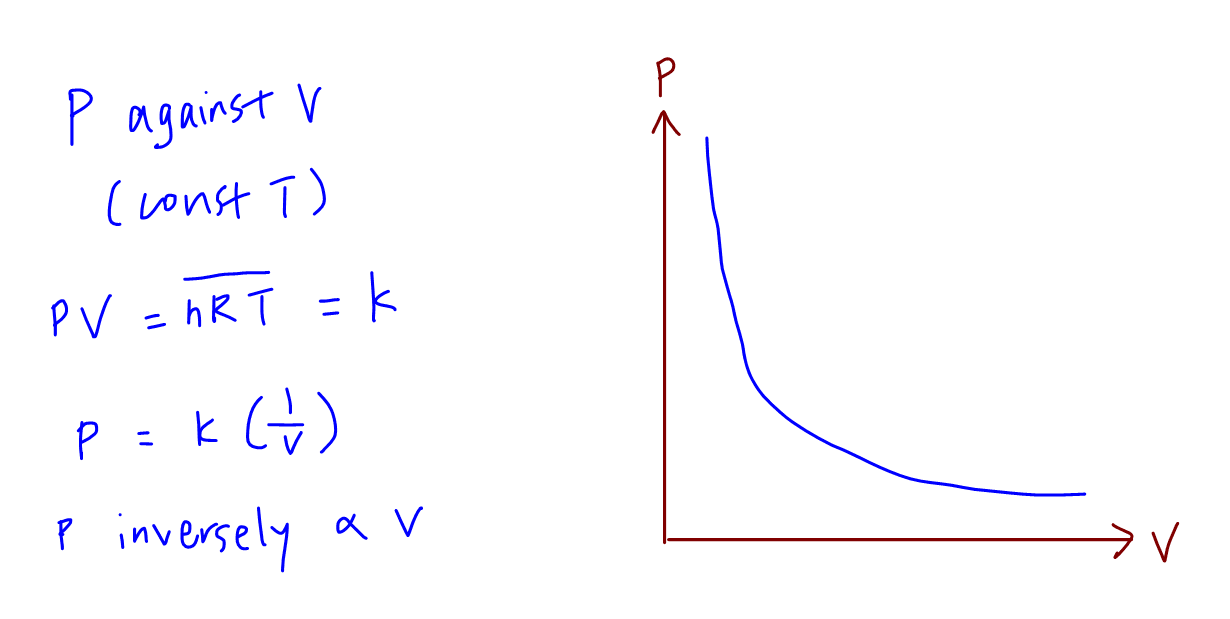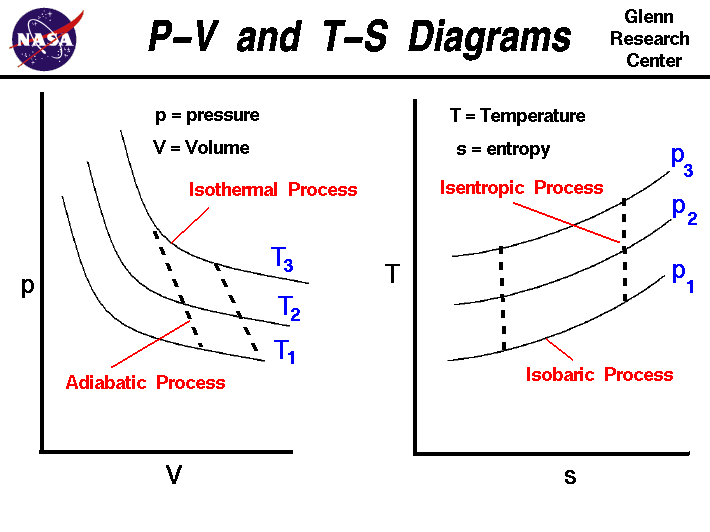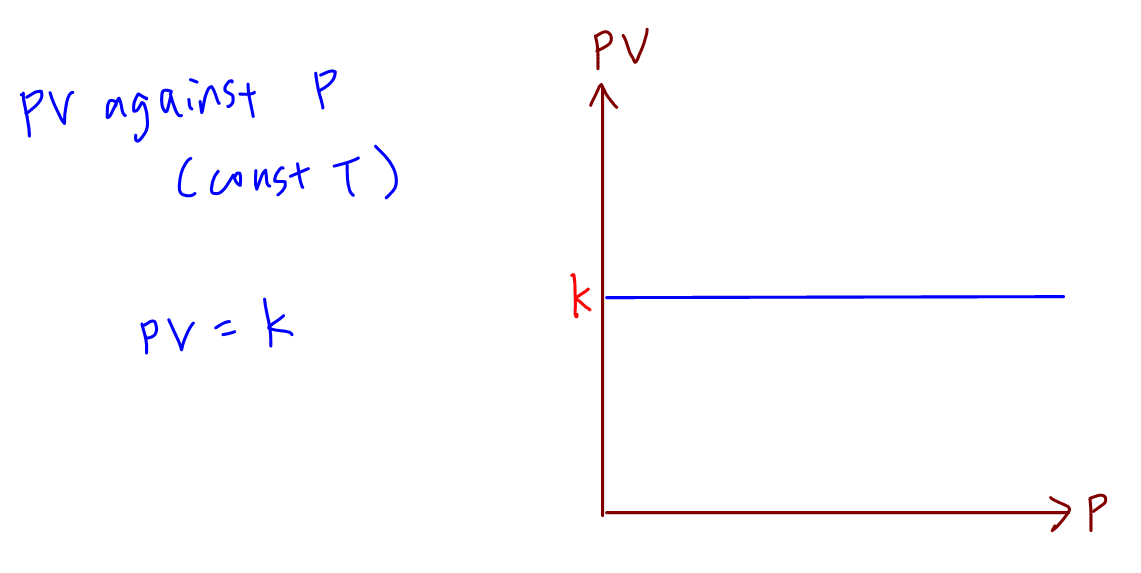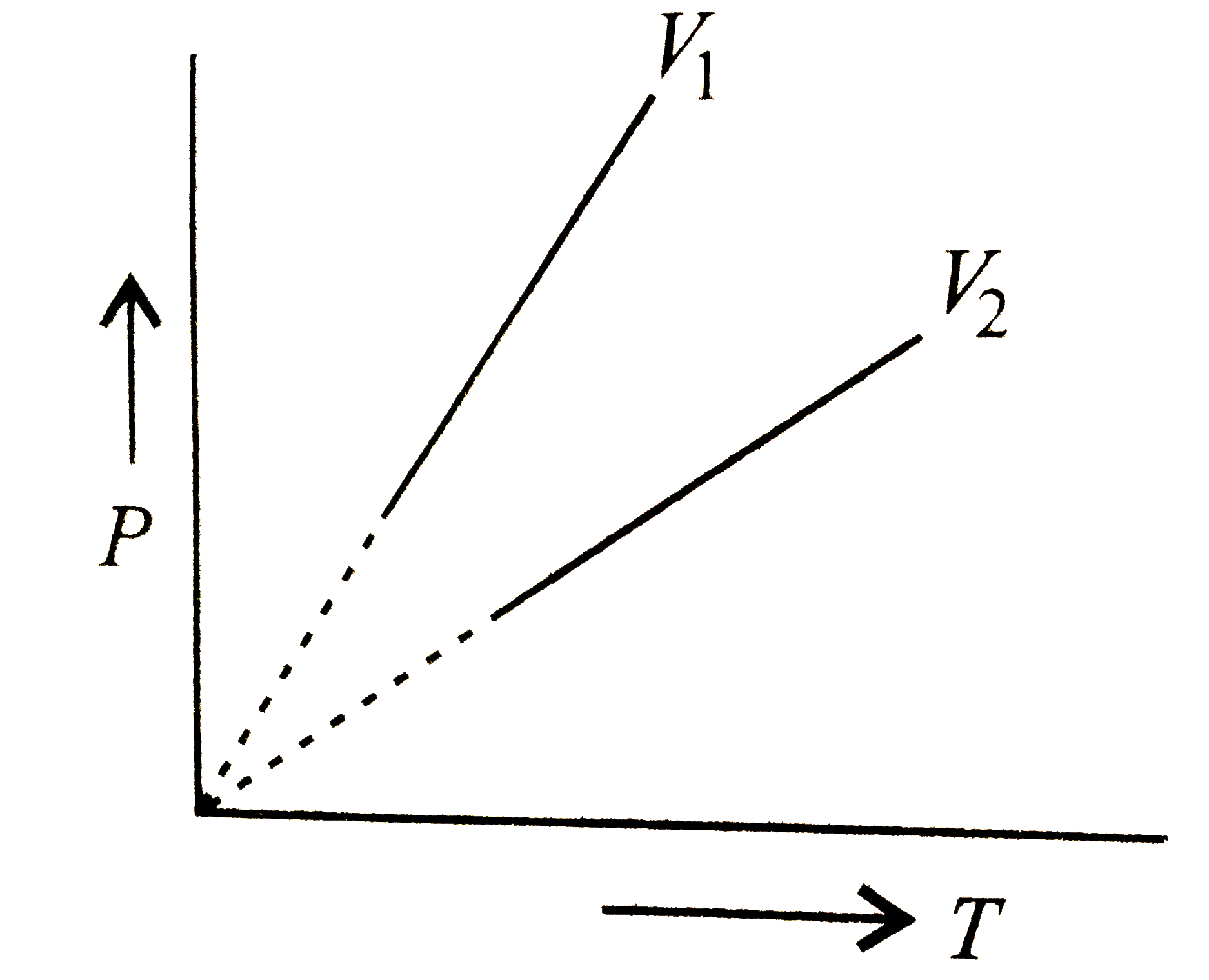
A plot of P vs T for a given mass of gas at constant volume is a straight line . P vs T at constant volumes V(1) and V(2) for an ideal

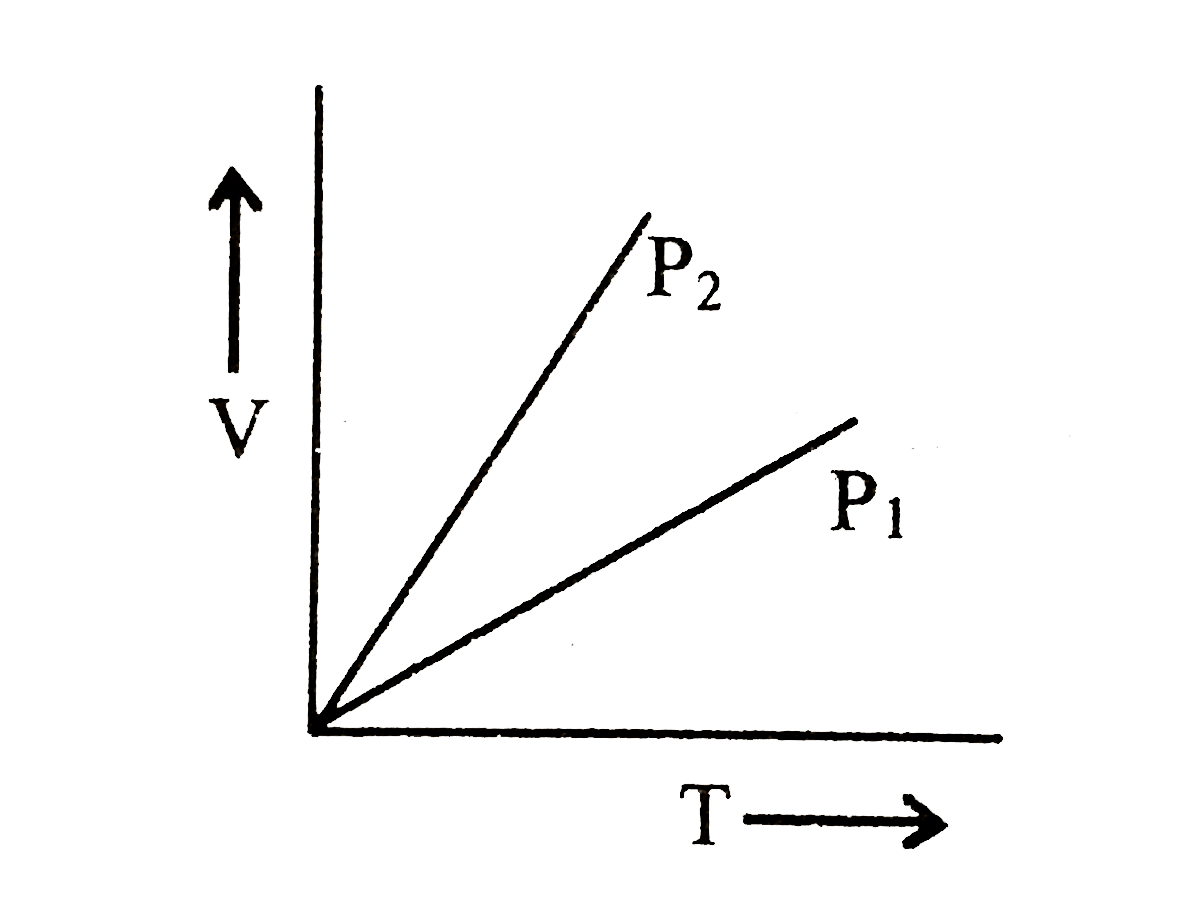
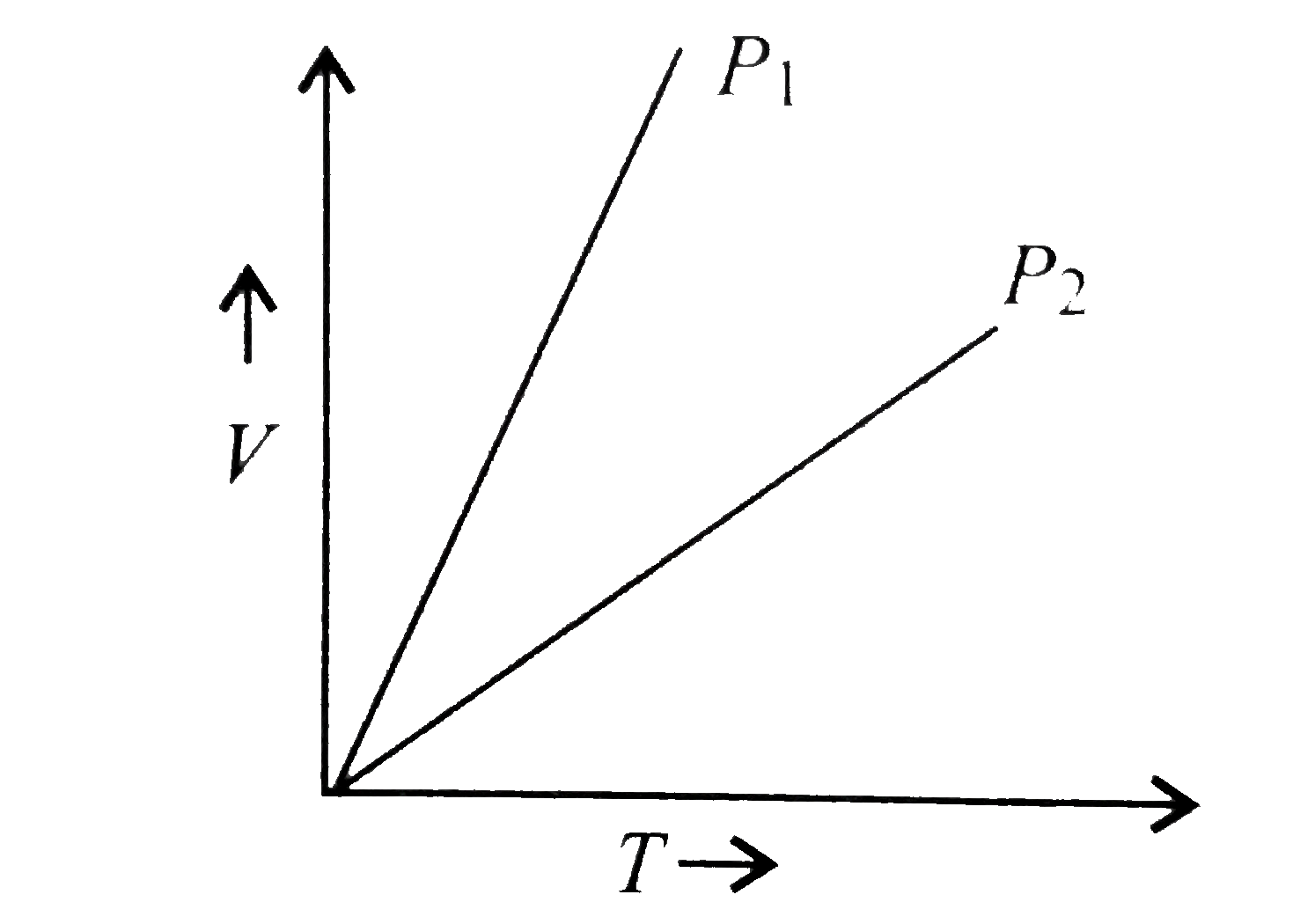
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line - YouTube
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1.

A plot of P vs T for a given mass of gas of constant volume is a straight line. P vs T at constant volumes V1 and V2 for an ideal gas
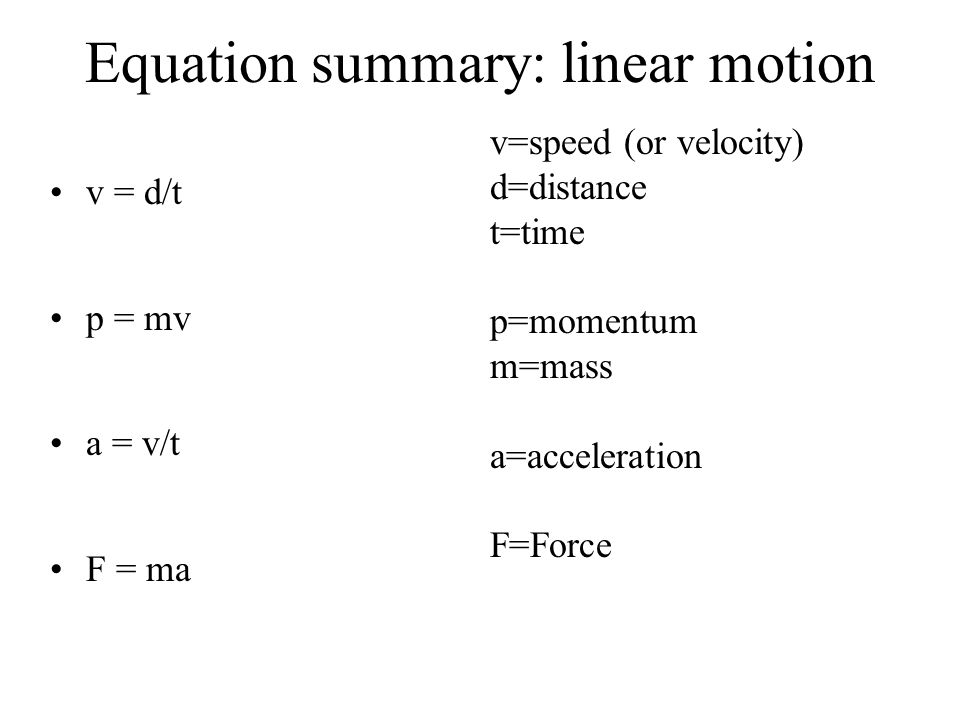
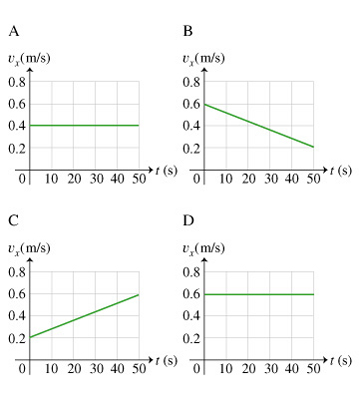
Equation summary: linear motion v = d/t p = mv a = v/t F = ma v=speed (or velocity) d=distance t=time p=momentum m=mass a=acceleration F=Force. - ppt download

V versus T curves at constant pressure P1 and P2 for an ideal gas as shown in figure. HereSelect one:a)P1 > P2b)P1 ≤ P2c)P1 = P2d)P1 < P2Correct answer is option 'A'.