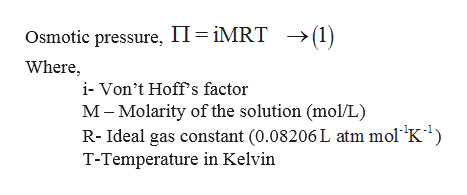
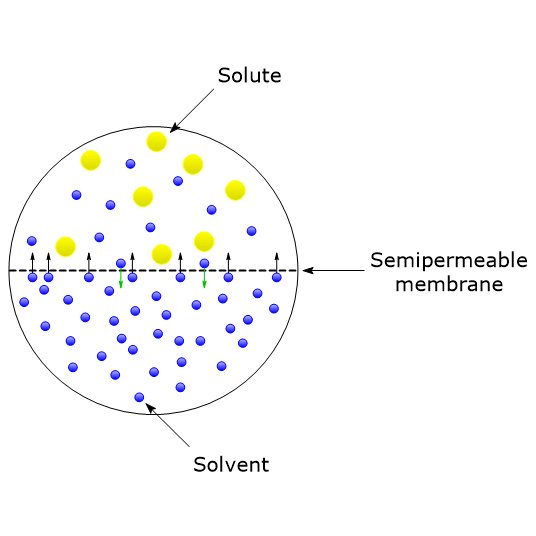
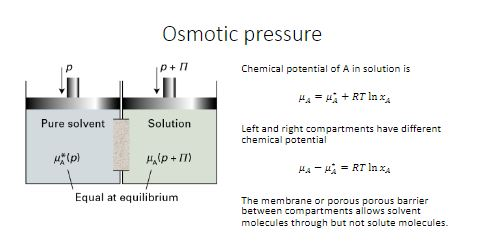
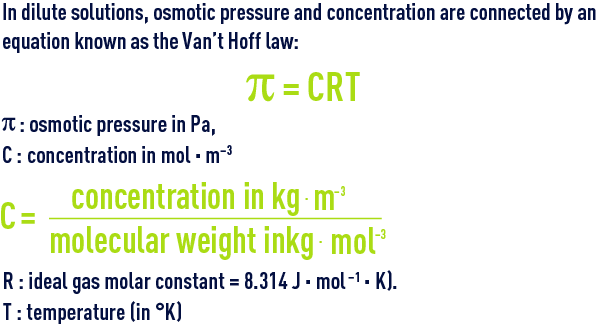
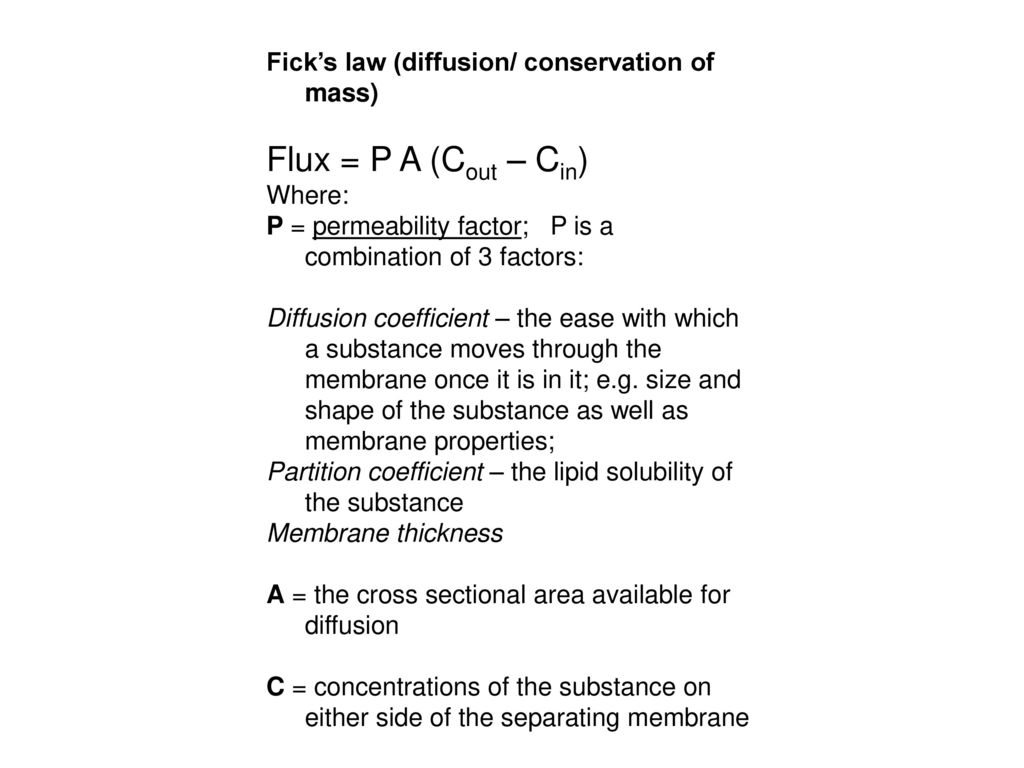
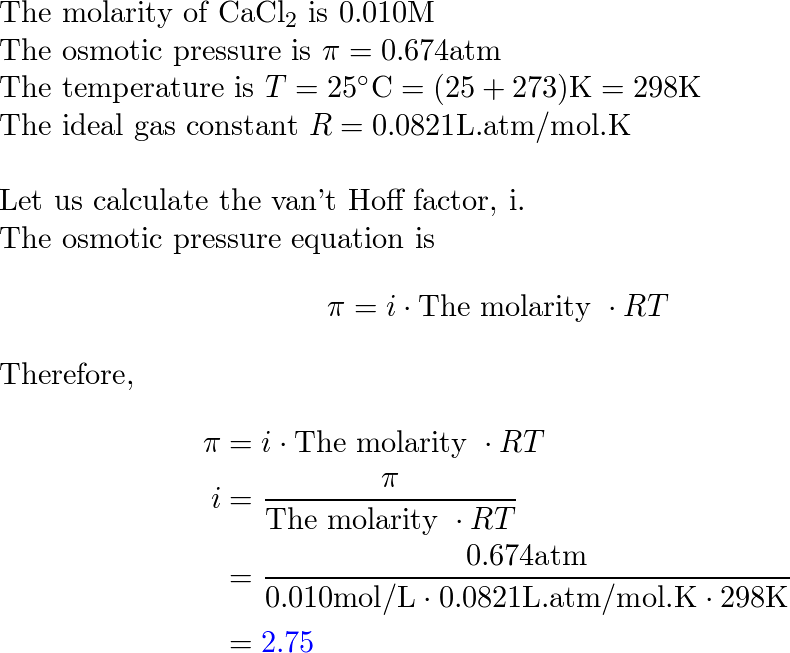
Vant Hoff formula - For calculation of Osmotic pressure ... ( Note: Maximum contribution of Plasma osmolarity is by sodi… | Osmotic pressure, Gas constant, Pressure

SOLVED: Membrane Separation II Reverse Osmosis (a) Derive van Hoff' s equation (osmotic pressure T = cRT) (b) feed solution containing Sg NaCI/L (p-1OOlkg/m ) is purified using reverse osmosis system: Water

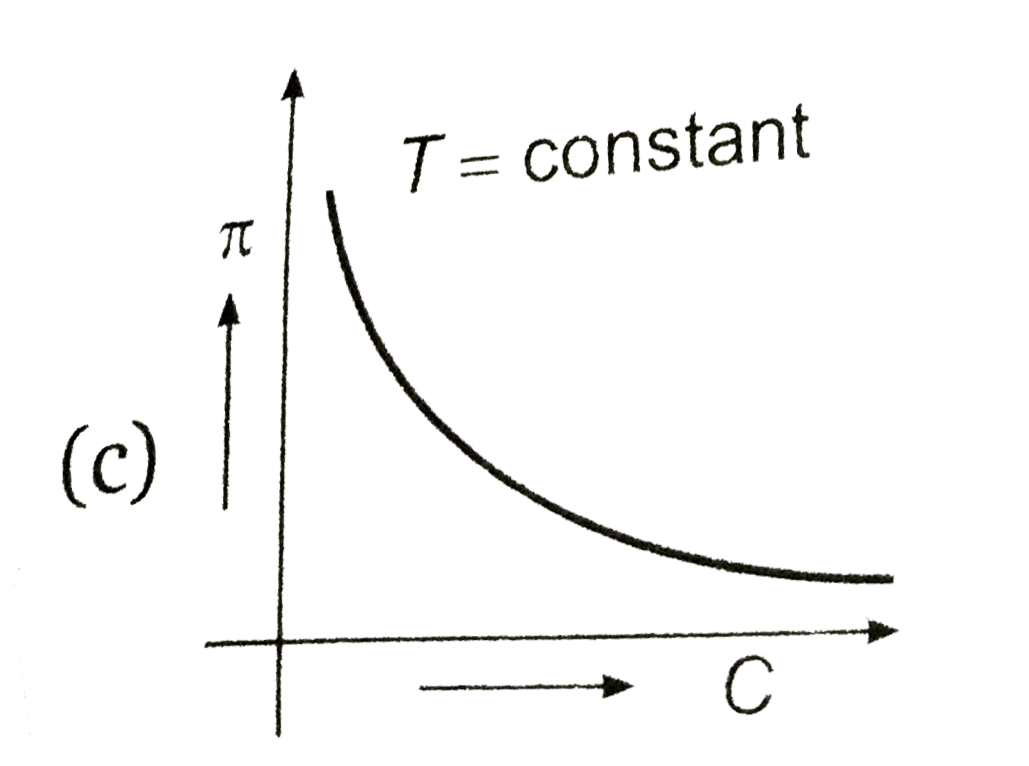
PPT - Osmotic pressure – van't Hoff equation : = g C R T Where: - osmotic pressure (atm or mm Hg) PowerPoint Presentation - ID:4239208