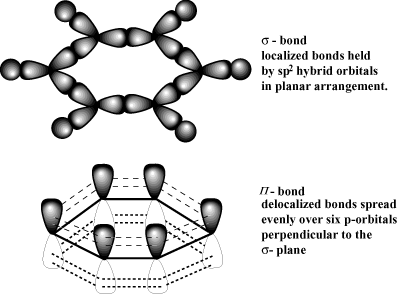
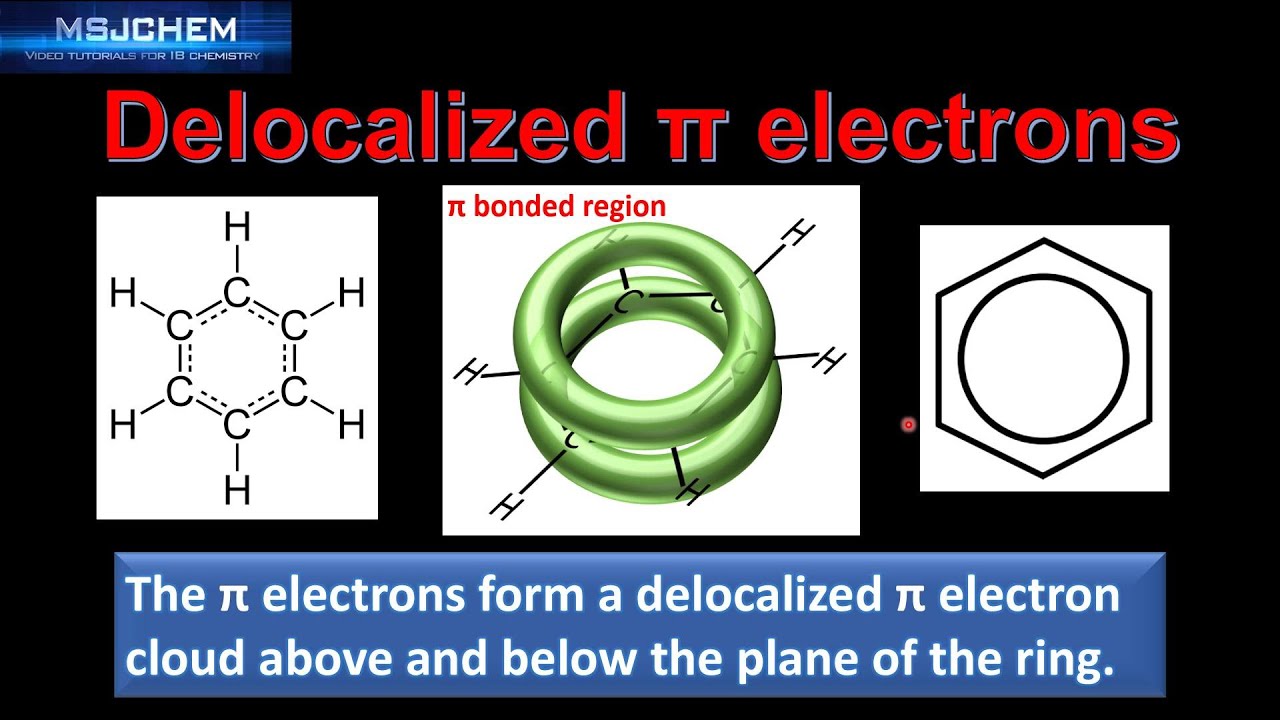
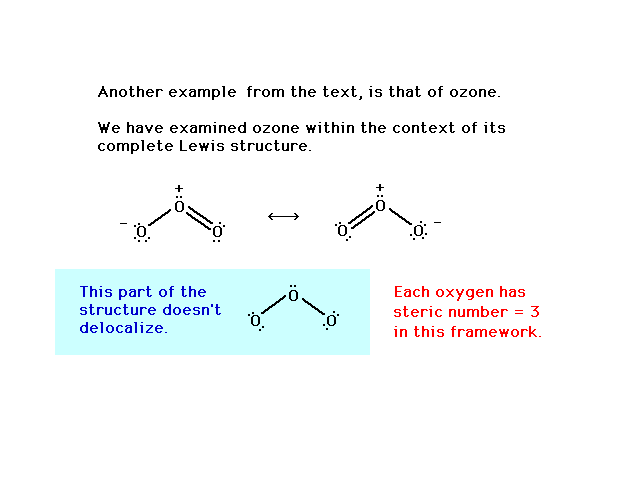
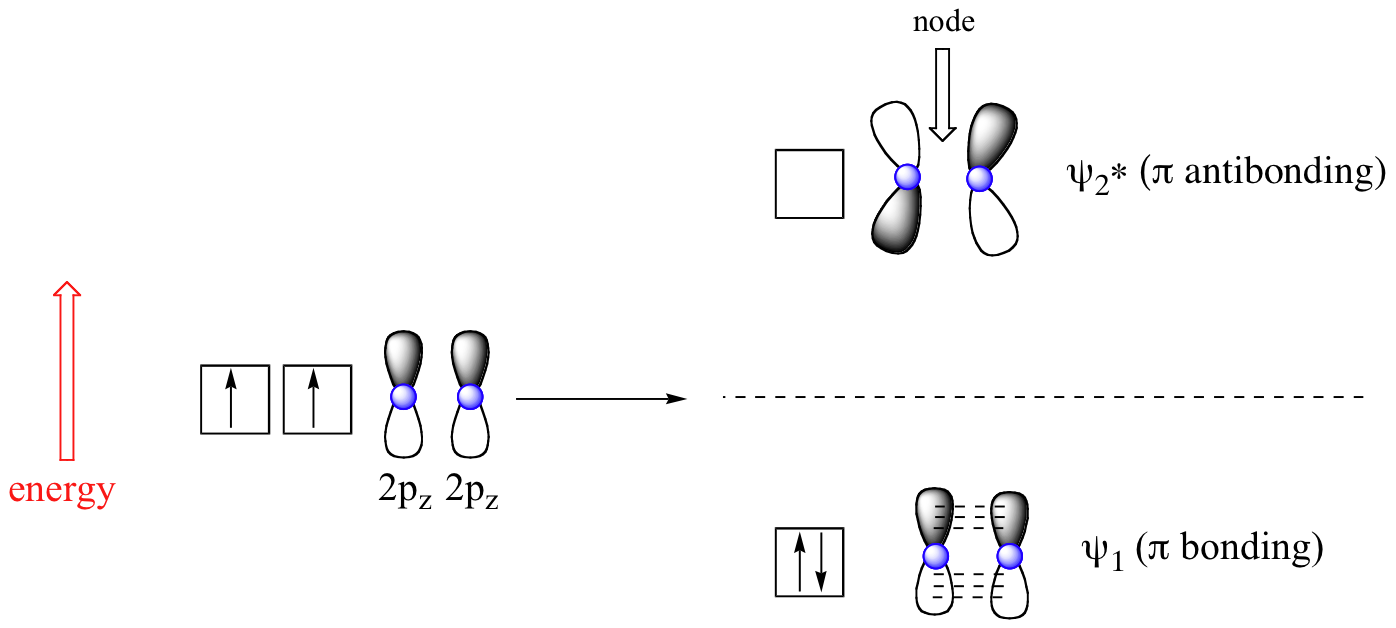
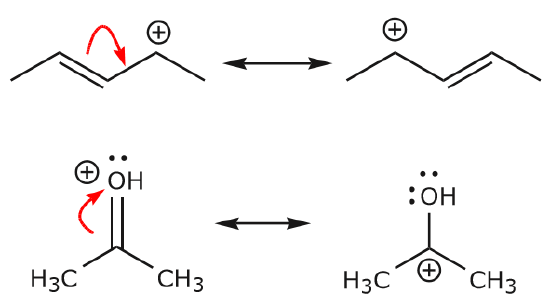
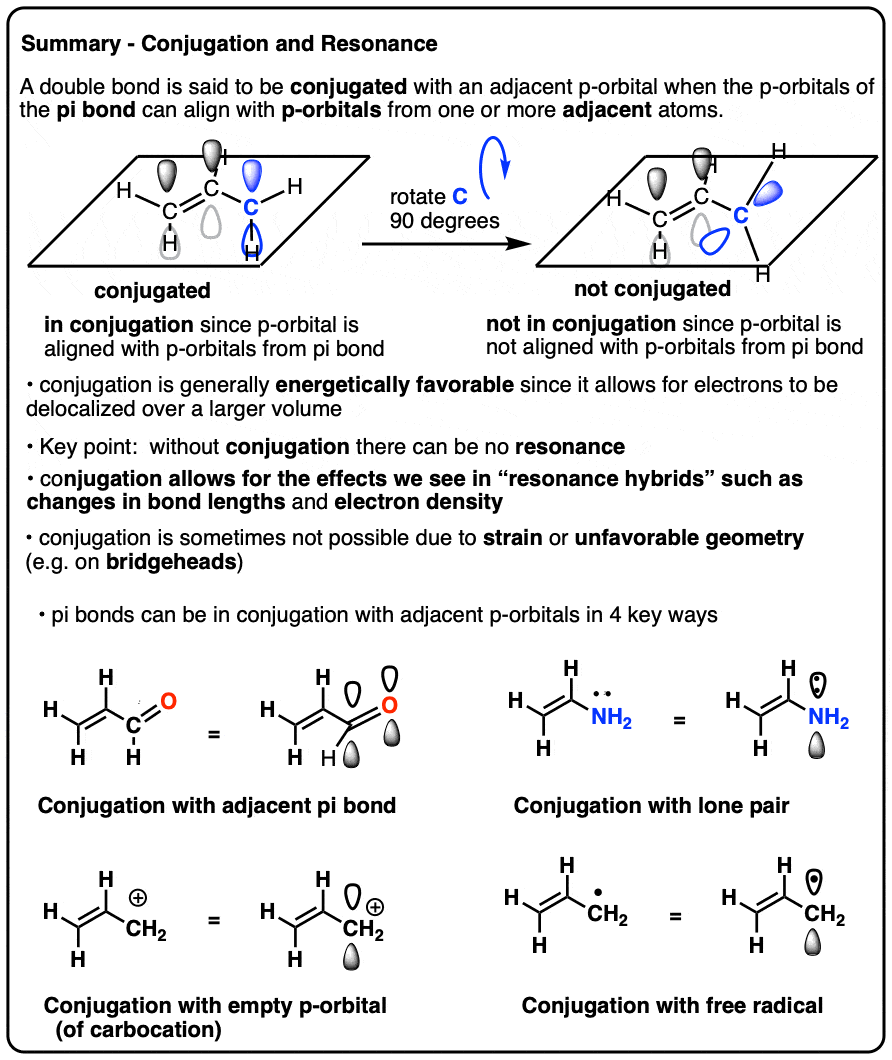
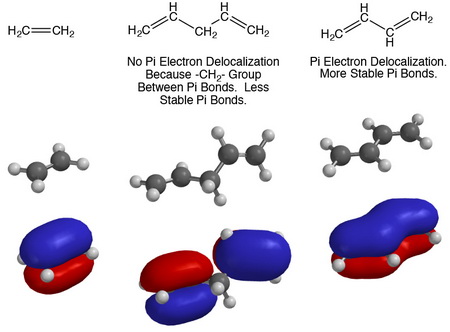
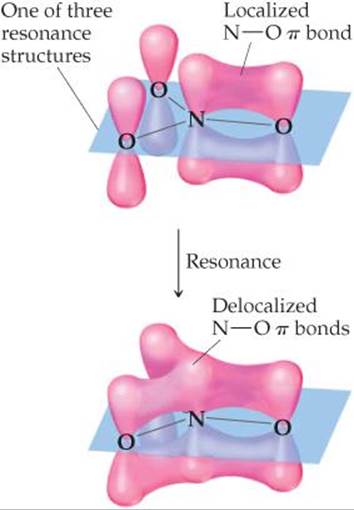
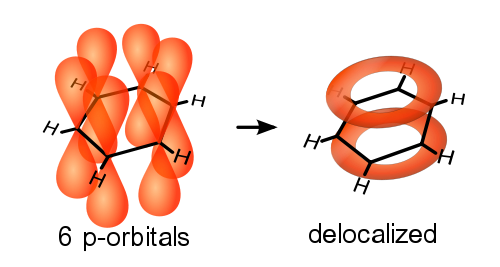A delocalised electron is an electron in an atom, ion, or molecule that is not connected to a single atom or covalent bond.

A delocalised electron is an electron in an atom, ion, or molecule that is not connected to a single atom or covalent bond.